Potassium Hydroxide Zinc Chloride Reaction . \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l). Here is an example, using mg 2+ :. The balanced equation will be calculated along. Web the balanced chemical equation for the reaction of zinc with potassium hydroxide solution is zn(s) + 2koh (aq)⇢ k 2 zno 2. Web do you want to learn how to write molecular, complete ionic, and. Web zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Web a rubidium hydroxide and cobalt(ii) chloride are strong electrolytes, so when aqueous solutions of these compounds are mixed,. Web the resulting hydroxide ions can participate in precipitation reactions. Web enter an equation of an ionic chemical equation and press the balance button. Web to start, write out the unbalanced equation for potassium permanganate (kmno4) + hydrogen. Web zncl2 + koh = zn (oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous zinc chloride [zncl 2].
from www.bartleby.com
Web enter an equation of an ionic chemical equation and press the balance button. Web a rubidium hydroxide and cobalt(ii) chloride are strong electrolytes, so when aqueous solutions of these compounds are mixed,. Web to start, write out the unbalanced equation for potassium permanganate (kmno4) + hydrogen. Web do you want to learn how to write molecular, complete ionic, and. Web the balanced chemical equation for the reaction of zinc with potassium hydroxide solution is zn(s) + 2koh (aq)⇢ k 2 zno 2. Web the resulting hydroxide ions can participate in precipitation reactions. The balanced equation will be calculated along. Web zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l). Web zncl2 + koh = zn (oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous zinc chloride [zncl 2].
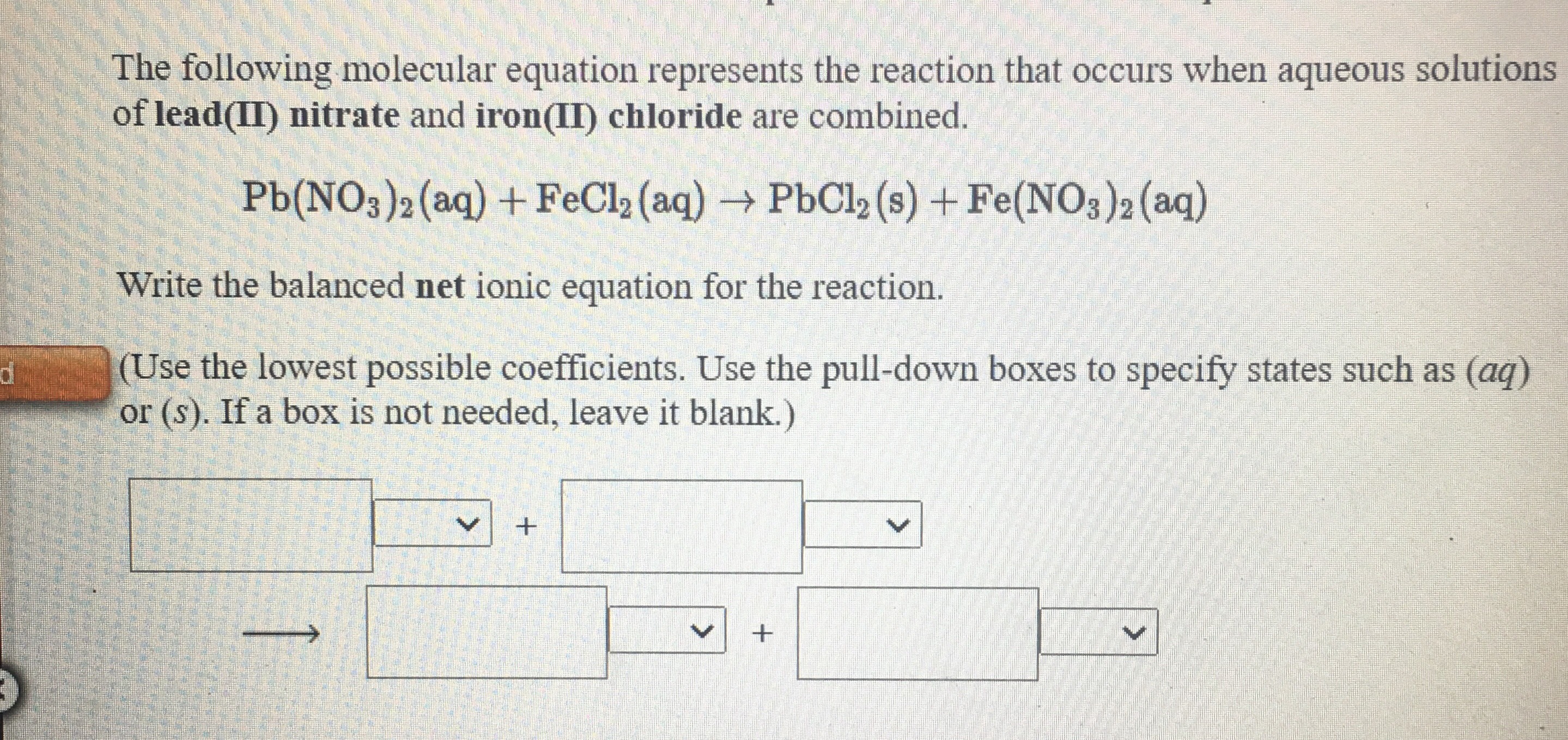
Answered When aqueous solutions of iron(II)… bartleby
Potassium Hydroxide Zinc Chloride Reaction Here is an example, using mg 2+ :. Web zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Web a rubidium hydroxide and cobalt(ii) chloride are strong electrolytes, so when aqueous solutions of these compounds are mixed,. Web zncl2 + koh = zn (oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous zinc chloride [zncl 2]. Web the resulting hydroxide ions can participate in precipitation reactions. Web enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along. Web to start, write out the unbalanced equation for potassium permanganate (kmno4) + hydrogen. Web the balanced chemical equation for the reaction of zinc with potassium hydroxide solution is zn(s) + 2koh (aq)⇢ k 2 zno 2. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l). Web do you want to learn how to write molecular, complete ionic, and. Here is an example, using mg 2+ :.
From www.myxxgirl.com
Hydrochloric Acid And Potassium Hydroxide My XXX Hot Girl Potassium Hydroxide Zinc Chloride Reaction Web enter an equation of an ionic chemical equation and press the balance button. Web zncl2 + koh = zn (oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous zinc chloride [zncl 2]. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l). Web do you want to learn how to write molecular, complete ionic, and. Web to start, write. Potassium Hydroxide Zinc Chloride Reaction.
From www.bartleby.com
Answered Write a balanced chemical equation… bartleby Potassium Hydroxide Zinc Chloride Reaction Web enter an equation of an ionic chemical equation and press the balance button. Here is an example, using mg 2+ :. Web zncl2 + koh = zn (oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous zinc chloride [zncl 2]. Web to start, write out the unbalanced equation for potassium permanganate (kmno4) + hydrogen.. Potassium Hydroxide Zinc Chloride Reaction.
From www.toppr.com
What happen whenPotassium ferrocyanide solution is added to a solution Potassium Hydroxide Zinc Chloride Reaction Here is an example, using mg 2+ :. Web the balanced chemical equation for the reaction of zinc with potassium hydroxide solution is zn(s) + 2koh (aq)⇢ k 2 zno 2. Web the resulting hydroxide ions can participate in precipitation reactions. Web zncl2 + koh = zn (oh)2 + kcl is a double displacement (metathesis) reaction where one mole of. Potassium Hydroxide Zinc Chloride Reaction.
From melscience.com
Reactions of potassium and potassium hydroxide MEL Chemistry Potassium Hydroxide Zinc Chloride Reaction Web enter an equation of an ionic chemical equation and press the balance button. Web the resulting hydroxide ions can participate in precipitation reactions. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l). The balanced equation will be calculated along. Web to start, write out the unbalanced equation for potassium permanganate (kmno4) + hydrogen. Web the balanced chemical equation for the reaction of zinc. Potassium Hydroxide Zinc Chloride Reaction.
From www.bartleby.com
Answered When aqueous solutions of iron(II)… bartleby Potassium Hydroxide Zinc Chloride Reaction Web enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along. Web the resulting hydroxide ions can participate in precipitation reactions. Web zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Web the balanced chemical equation for the reaction of zinc with potassium hydroxide solution is zn(s) +. Potassium Hydroxide Zinc Chloride Reaction.
From insende.netlify.app
30+ Hydrochloric Acid And Sodium Hydroxide Equation Insende Potassium Hydroxide Zinc Chloride Reaction Web a rubidium hydroxide and cobalt(ii) chloride are strong electrolytes, so when aqueous solutions of these compounds are mixed,. Web the balanced chemical equation for the reaction of zinc with potassium hydroxide solution is zn(s) + 2koh (aq)⇢ k 2 zno 2. Web zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Web zncl2 + koh = zn. Potassium Hydroxide Zinc Chloride Reaction.
From www.chegg.com
Solved What Would Be The Proper Mechanism Of The Reaction... Potassium Hydroxide Zinc Chloride Reaction Web to start, write out the unbalanced equation for potassium permanganate (kmno4) + hydrogen. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l). The balanced equation will be calculated along. Web do you want to learn how to write molecular, complete ionic, and. Here is an example, using mg 2+ :. Web the balanced chemical equation for the reaction of zinc with potassium hydroxide. Potassium Hydroxide Zinc Chloride Reaction.
From www.nagwa.com
Question Video Writing the Balanced Net Ionic Equation for the Potassium Hydroxide Zinc Chloride Reaction Web a rubidium hydroxide and cobalt(ii) chloride are strong electrolytes, so when aqueous solutions of these compounds are mixed,. Here is an example, using mg 2+ :. Web the balanced chemical equation for the reaction of zinc with potassium hydroxide solution is zn(s) + 2koh (aq)⇢ k 2 zno 2. Web zncl2 + koh = zn (oh)2 + kcl is. Potassium Hydroxide Zinc Chloride Reaction.
From thaipurescience.com
Potassium hydroxide Thai Pure Science Potassium Hydroxide Zinc Chloride Reaction Web a rubidium hydroxide and cobalt(ii) chloride are strong electrolytes, so when aqueous solutions of these compounds are mixed,. The balanced equation will be calculated along. Here is an example, using mg 2+ :. Web zncl2 + koh = zn (oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous zinc chloride [zncl 2]. Web enter. Potassium Hydroxide Zinc Chloride Reaction.
From www.k2ksigns.com.au
Potassium Hydroxide Solutions K2K Signs Potassium Hydroxide Zinc Chloride Reaction Web the balanced chemical equation for the reaction of zinc with potassium hydroxide solution is zn(s) + 2koh (aq)⇢ k 2 zno 2. Web a rubidium hydroxide and cobalt(ii) chloride are strong electrolytes, so when aqueous solutions of these compounds are mixed,. Web enter an equation of an ionic chemical equation and press the balance button. Web zncl2 + koh. Potassium Hydroxide Zinc Chloride Reaction.
From www.youtube.com
How to Balance K + HCl = KCl + H2 Potassium + Hydrochloric acid Potassium Hydroxide Zinc Chloride Reaction Web the balanced chemical equation for the reaction of zinc with potassium hydroxide solution is zn(s) + 2koh (aq)⇢ k 2 zno 2. Web do you want to learn how to write molecular, complete ionic, and. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l). Web enter an equation of an ionic chemical equation and press the balance button. Web the resulting hydroxide ions. Potassium Hydroxide Zinc Chloride Reaction.
From www.numerade.com
SOLVED A 340cm3 of 1.5mol.dm^3 potassium hydroxide solution reacts Potassium Hydroxide Zinc Chloride Reaction Web zncl2 + koh = zn (oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous zinc chloride [zncl 2]. Web the resulting hydroxide ions can participate in precipitation reactions. Web a rubidium hydroxide and cobalt(ii) chloride are strong electrolytes, so when aqueous solutions of these compounds are mixed,. Web zinc(ii) ion reacts with aqueous ammonia. Potassium Hydroxide Zinc Chloride Reaction.
From www.chemicalslearning.com
What is the Reaction of Magnesium Chloride and Sodium Hydroxide? Potassium Hydroxide Zinc Chloride Reaction Web do you want to learn how to write molecular, complete ionic, and. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l). Here is an example, using mg 2+ :. The balanced equation will be calculated along. Web zncl2 + koh = zn (oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous zinc chloride [zncl 2]. Web enter an. Potassium Hydroxide Zinc Chloride Reaction.
From www.researchgate.net
Reaction scheme. Reagents and conditions (i) potassium hydroxide Potassium Hydroxide Zinc Chloride Reaction \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l). Web do you want to learn how to write molecular, complete ionic, and. The balanced equation will be calculated along. Web enter an equation of an ionic chemical equation and press the balance button. Web the resulting hydroxide ions can participate in precipitation reactions. Web to start, write out the unbalanced equation for potassium permanganate. Potassium Hydroxide Zinc Chloride Reaction.
From www.bartleby.com
Answered Write the balanced NET ionic equation… bartleby Potassium Hydroxide Zinc Chloride Reaction Web the balanced chemical equation for the reaction of zinc with potassium hydroxide solution is zn(s) + 2koh (aq)⇢ k 2 zno 2. Here is an example, using mg 2+ :. Web a rubidium hydroxide and cobalt(ii) chloride are strong electrolytes, so when aqueous solutions of these compounds are mixed,. Web to start, write out the unbalanced equation for potassium. Potassium Hydroxide Zinc Chloride Reaction.
From www.toppr.com
Identify the type of reactions taking place in each of the following Potassium Hydroxide Zinc Chloride Reaction Web enter an equation of an ionic chemical equation and press the balance button. Web do you want to learn how to write molecular, complete ionic, and. Here is an example, using mg 2+ :. Web the resulting hydroxide ions can participate in precipitation reactions. Web zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: The balanced equation. Potassium Hydroxide Zinc Chloride Reaction.
From schoolworkhelper.net
Single Displacement Reactions Lab Explained SchoolWorkHelper Potassium Hydroxide Zinc Chloride Reaction \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l). Web a rubidium hydroxide and cobalt(ii) chloride are strong electrolytes, so when aqueous solutions of these compounds are mixed,. Here is an example, using mg 2+ :. Web the resulting hydroxide ions can participate in precipitation reactions. Web to start, write out the unbalanced equation for potassium permanganate (kmno4) + hydrogen. Web zinc(ii) ion reacts. Potassium Hydroxide Zinc Chloride Reaction.
From www.numerade.com
SOLVED Write an unbalanced equation to represent each of the following Potassium Hydroxide Zinc Chloride Reaction Web zncl2 + koh = zn (oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous zinc chloride [zncl 2]. Web a rubidium hydroxide and cobalt(ii) chloride are strong electrolytes, so when aqueous solutions of these compounds are mixed,. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l). The balanced equation will be calculated along. Web the resulting hydroxide ions. Potassium Hydroxide Zinc Chloride Reaction.